Phospholine Iodide® for Uncontrolled IOP (echothiophate iodide for ophthalmic solution)
A valuable therapy option for glaucoma patients with uncontrolled IOP
IOP decreased in 31 of 32 eyes in a retrospective study of children with aphakic or pseudophakic glaucoma1
- Mean baseline IOP decreased from 29.1 ± 5.3 mm Hg to 19.6 ± 6.7 mm Hg in patients who were initially taking at least one other medication
- 4 eyes eventually required surgery for uncontrolled IOP
- 6 eyes had IOP spikes that could not be controlled with other medications when Phospholine Iodide was discontinued due to product unavailability
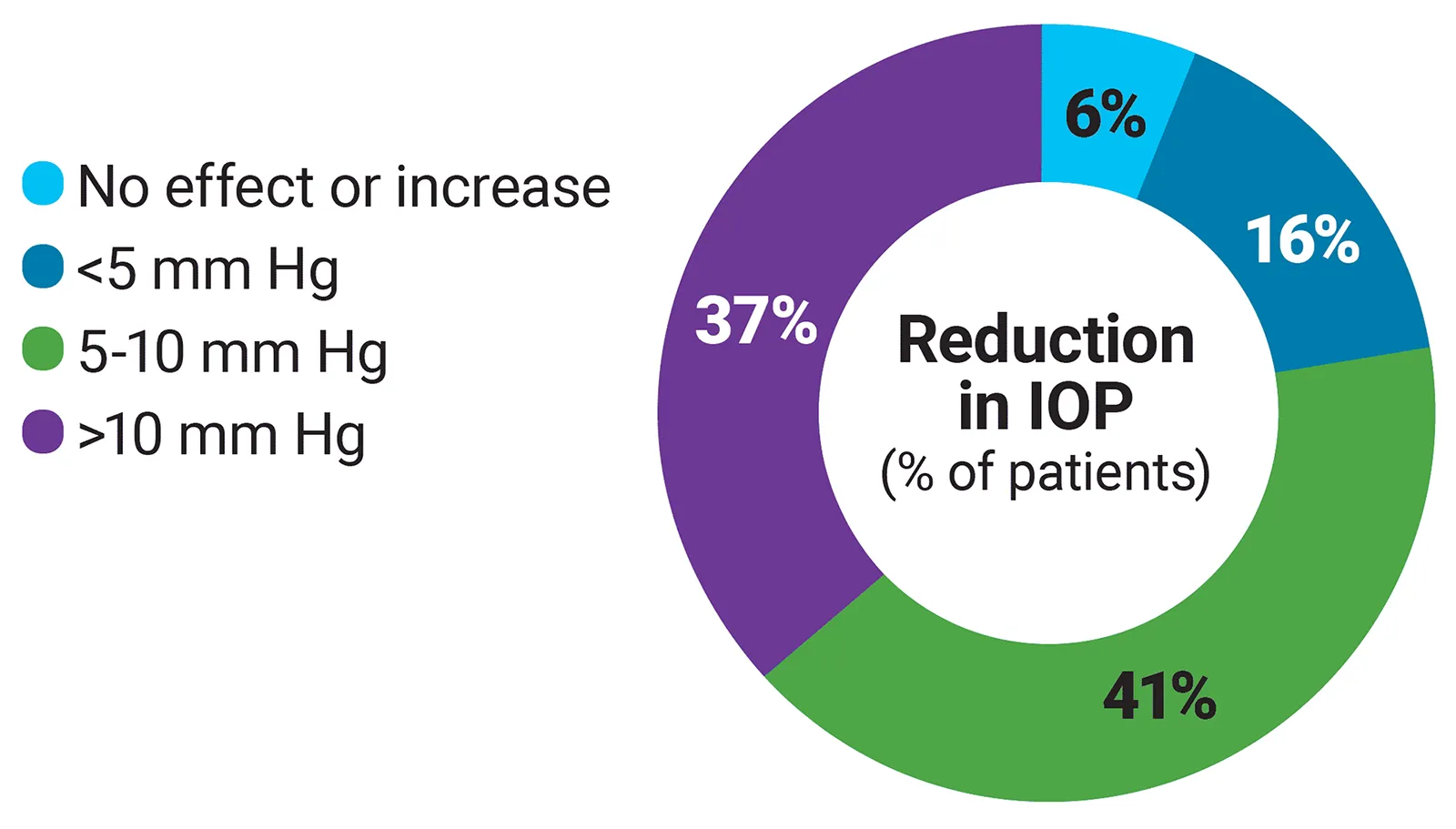
A retrospective study of 32 eyes (27 aphakic and 5 pseudophakic) in 21 children aged 40 days to 12 years with echothiophate iodide exposure after infantile cataract surgery. Glaucoma was defined as IOP >21 mm Hg with one or more of the following: corneal enlargement; asymmetrical progressive myopic shift coupled with enlargement of the corneal diameter and/or axial length; increased optic nerve cupping. Phospholine Iodide dosage was 0.125% BID for a mean of 3.5 years.
IOP=intraocular pressure.
IOP decreased in 23 of 24 eyes in a retrospective study of elderly patients with pseudophakic glaucoma2
- Mean baseline IOP decreased from 29.54 ± 7.9 mm Hg to 17.8 ± 5.4 mm Hg after addition of Phospholine Iodide to maximal medical therapy (average of 2.8 ± 1.1 medications)
- While IOP was reduced in the majority of eyes, 4 eyes remained uncontrolled after addition of Phospholine Iodide
- Mean IOP increased to 27.4 ± 8.2 mm Hg when Phospholine Iodide was discontinued due to product unavailability

A retrospective study of 24 eyes in 24 elderly patients (mean age 74 years; 11 men and 13 women) with uncontrolled IOP despite maximal medical therapy, including prostaglandin analogs, alpha-2 agonists, and topical carbonic anhydrase inhibitors. Exclusions: Patients with ocular inflammation, high myopia, conditions affecting applanation tonometry, laser or intraocular surgery within 12 months, contraindication to cholinergic drugs, initiation of systemic treatment with medications that may influence IOP or corticosteroids, and any other alteration in medication. Phospholine Iodide dosage was 0.125% 1 drop BID. The average follow-up was 11.2 months.
These retrospective studies had multiple limitations, including small sample size and lack of a control group and standardized initial treatment.
- Kraus, CL, Trivedi, RH, Wilson, ME. Intraocular pressure control with echothiophate iodide in children’s eyes with glaucoma after cataract extraction. JAAPOS. 2015 Apr;19(2):116-8.el. doi: 10.1016/j.jaapos.2014.11.006.
- Schmidt, KG, Horowitz, Y, Buckman, G, et al. Lowering of IOP by echothiophate iodide in pseudophakic eyes with glaucoma. Curr Eye Res. 2010 Aug;35(8):698-702. doi: 10.3109/02713681003794076
